Abstract
Introduction: Thrombotic manifestations, such as deep vein thrombosis, pulmonary embolism, and rarely ischemic stroke, have been identified as disease complications of COVID-19. However, less is known about bleeding complications of COVID-19. While COVID-19-related intracerebral hemorrhage (ICH) has been described in the literature, the underlying risk factors are poorly understood.
Methods: We performed a retrospective observational study of consecutively admitted ICH patients in the Mount Sinai Health System in New York, NY. We compared demographic variables, medical comorbidities, in-hospital treatments, and time to diagnosis between COVID-19-positive and COVID-19-negative cohorts.
Results: Forty-seven patients were diagnosed with ICH, and of these, 31.9% (n=15) were COVID-19-positive. There were no significant differences in age, race-ethnicity, or cardiovascular risk factors. However, a higher proportion of patients with COVID-19 had a history of obesity, defined as BMI ≥ 30 (73.3% vs. 30.0%, p=0.01).
For the COVID-19-negative cohort mean days from presenting symptom onset to ICH diagnosis was 1.4 (95% CI -1.3-3.9). For the COVID-19-positive cohort, ICH was diagnosed an average of 7.9 days from hospital admission (95% CI 2.6-13.3) and 12.3 days from presenting symptom onset (95% CI 5.6-19.0). Of the COVID-19-negative patients, 94% had an admitting diagnosis of ICH, while only 40% of COVID-19-positive patients had an admitting diagnosis of ICH.
At the time of this study, the MSHS used a COVID-19 anticoagulation algorithm that recommended consideration of treatment-dose anticoagulation for patients with moderate or severe COVID-19. Patients with severe or worsening respiratory status (based on oxygen requirement, elevations in D-dimer, creatinine, or C-reactive protein) were recommended to be treated with moderate dose anticoagulation (enoxaparin 1 mg/kg every 24 hours) and the recommendation for all COVID-19 ICU patients was treatment dose anticoagulation (enoxaparin 1 mg/kg every 12 hours or apixaban).
Forty percent (40%) of patients in the COVID-19-positive cohort were on full-dose anticoagulation at the time of ICH compared to <10% in the COVID-19-negative cohort. Of the COVID-19-positive cohort on full-dose anticoagulation, one-third were on full-dose anticoagulation due to institutional COVID-19 treatment protocols. The rest were treated with anticoagulation for other indications, most commonly atrial fibrillation. Nearly 60% of COVID-19 patients who suffered ICH were not taking full dose anticoagulation.
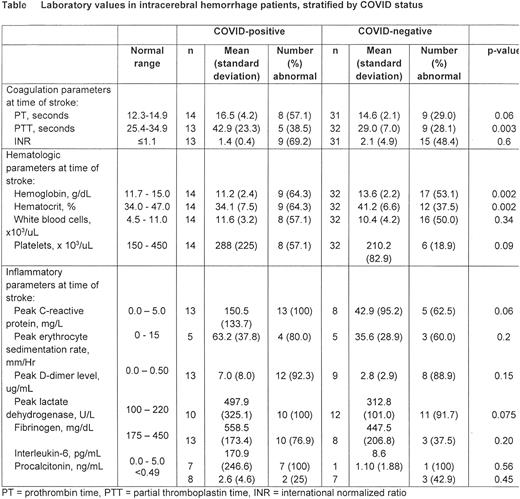
Table 1 summarizes laboratory values among the COVID-19-positive cohort compared to the COVID-19-negative cohort. At the time closest to ICH discovery, mean coagulation markers were slightly elevated (PT 16.5±4.2, PTT 42.9±23.3) compared to the COVID-19-negative cohort (PT 14.6±2.1, PTT 29.0±7.0, p=0.06 and 0.003 respectively), although INR did not significantly differ between the two groups (1.4±0.4 vs. 2.1±4.9). Hemoglobin, hematocrit, white blood cell, and platelet counts were abnormal in >50% of COVID-19-positive patients, compared to approximately 50% or less in COVID-19-negative patients. Inflammatory parameters were elevated in the majority of COVID-19 patients, including C-reactive protein, erythrocyte sedimentation rate, d-dimer, lactate dehydrogenase, fibrinogen, and interleukin-6. The peak CRP in COVID-19 patients (150.5±133.7) was markedly elevated compared to COVID-19-negative patients (42.9±95.2, p=0.06).
Conclusions: The admitting diagnosis and time to ICH diagnosis suggest that ICH in COVID-19 is not typically the presenting manifestation of the disease and is more commonly seen in severe disease requiring prolonged hospitalization. Patients with COVID-19 and ICH were more likely to be receiving full-dose anticoagulation than their COVID-19 negative counterparts. ICH in COVID-19 patients is potentially related to anticoagulation, not an underlying inflammatory state or consumptive coagulopathy, and underscores the need for careful consideration of anticoagulation in COVID-19 patients.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal